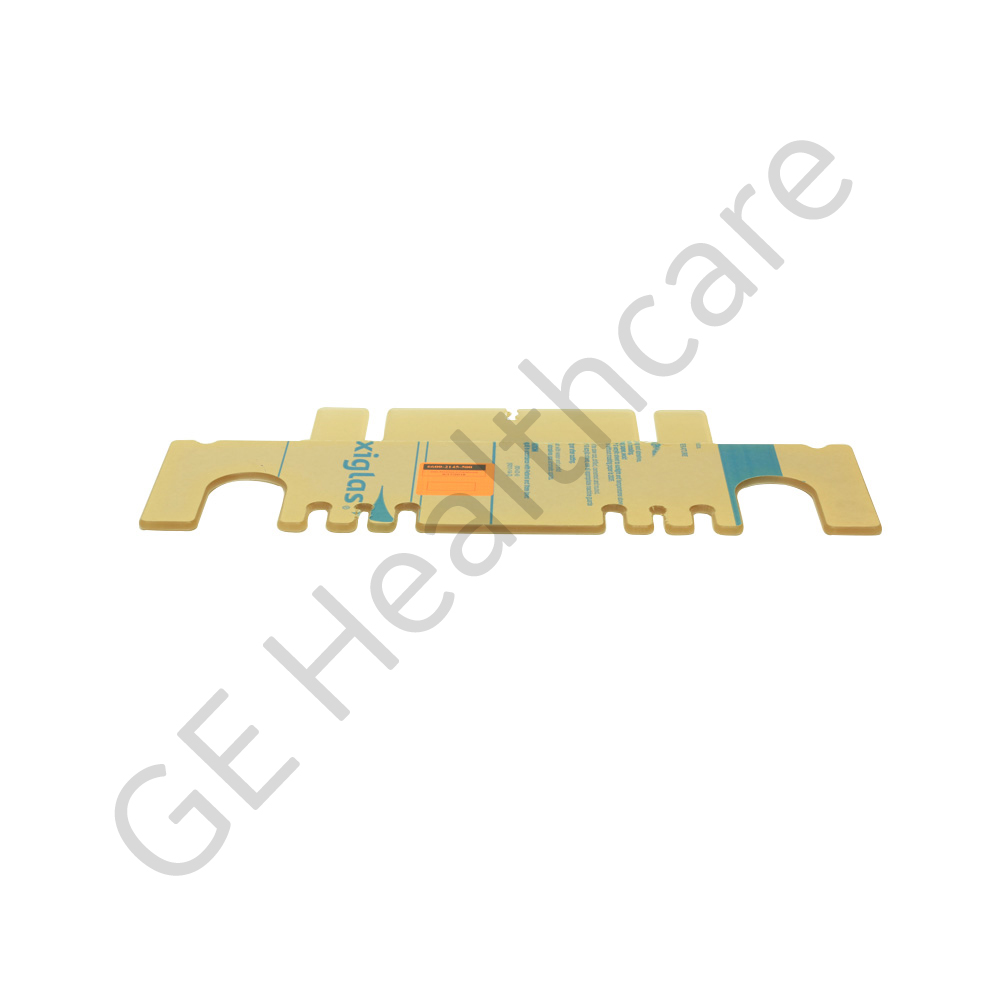
Door Tubing Management North LDR
| 6600-2145-500 | |
| Maternal Infant Care | |
| GE Sistemas Médicos de México S.A. de C.V | |
| GE HealthCare | |
Enter your approval number and submit to add item(s) to cart.
Please enter approval number
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without the
approval number, GE will contact you before your order
can be confirmed for shipment.
Select your approver's name and submit to add item(s) to your cart
Please Select Approver Name
OR
Don't know your approval number? Call 800-437-1171
Enter opt 1 for the first three prompts, and have your System ID available.
If you add item(s) to cart and submit your order without
selecting an approver, GE will contact you before your order
can be confirmed for shipment.
Features
- High quality north wall tubing management
- Smooth surface
- Excellent dimensional stability
- Excellent thermal resistance
Product Overview
The Door Tubing Management North LDR is a specially designed part intended for use in Panda™ iRes Warmer incubators and other medical equipment as applicable. The door wall is the sub component of the bedside panel assembly and attached north side tubing management of infant incubator. The wall plate is made of a high quality acrylic sheet material that has excellent optical characteristics, light stability, low internal stress levels, excellent chemical resistance and has flame retardant capability, which makes it well suited for many medical applications. The assembly is RoHS compliant and approved for todays safety standards. The product is tested to work at extreme atmospheric conditions. The products superior material quality offers reliable operation, efficient functioning and longer life span. The product is securely packaged inside a high quality packing box and is supported by bubble wrap or pads at the sides to avoid physical damage during transit.
Additional Features
- Lightweight and good rigidity
- Good optical characteristics
- Good impact and shear strength
- Water and solvent resistant
- Excellent tensile strength
- Chemical resistant
- Suitable for indoor and outdoor application
- Durable
- RoHS compliant




